Ubiquitin: beyond molecular tagging
Inspired by Nobel Prize laureate Dr. Aaron Ciechanover, whose pioneering work unveiled the intricate mechanisms of ubiquitin-mediated proteolysis.
Makliya Mamat / March 23, 2024
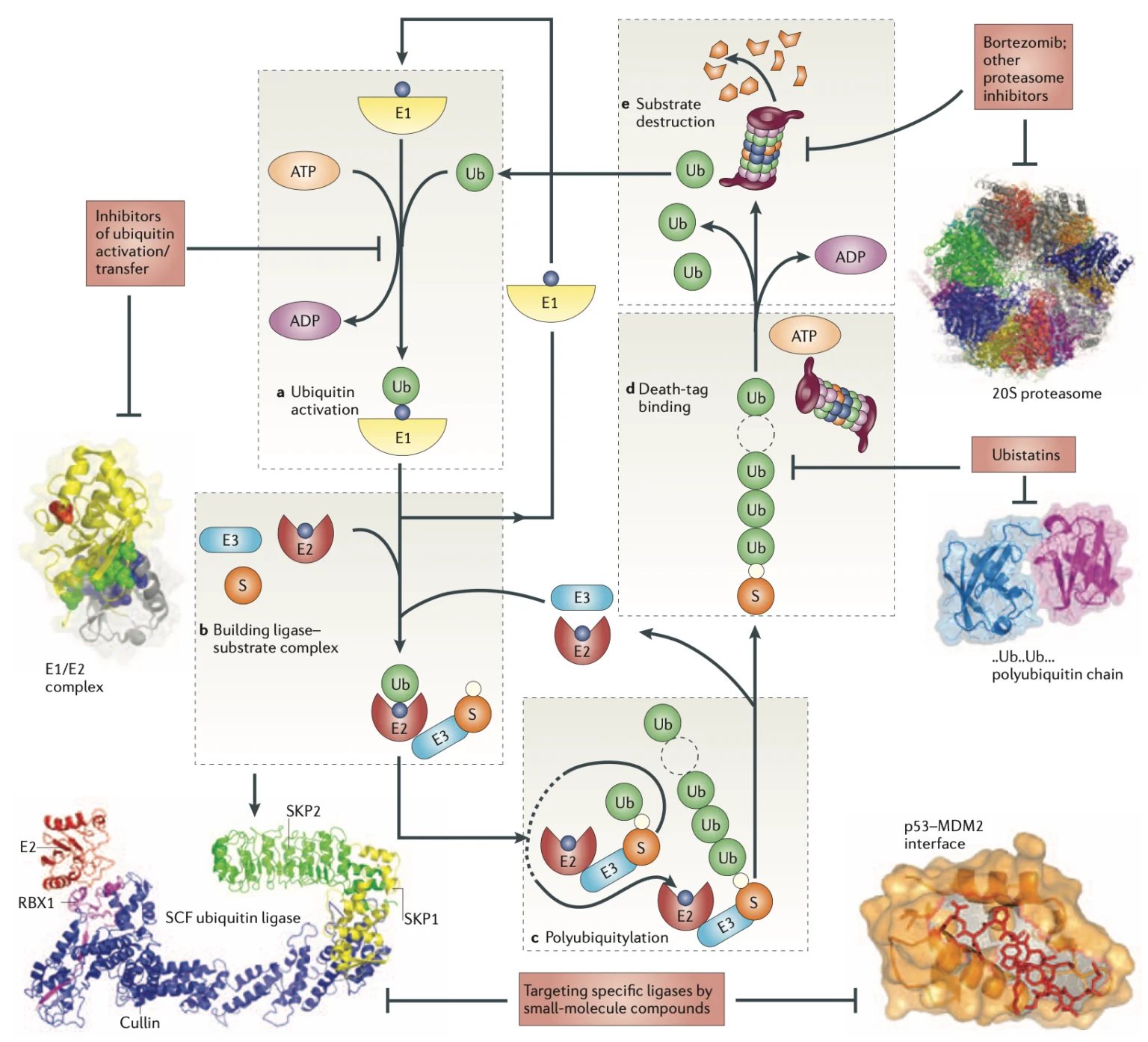
Ubiquitin, aptly named for its ubiquitous presence across all cells, serves as a molecular tagger, marking proteins for various fates within the cell. Its primary function lies in the regulation of protein degradation, a crucial aspect of cellular homeostasis. Through a process known as ubiquitination, ubiquitin molecules attach to target proteins, flagging them for degradation by the cell’s proteasome machinery. This mechanism ensures the timely removal of damaged or surplus proteins, thereby maintaining cellular integrity and function.
Nalepa, G., Rolfe, M. & Harper, J. Drug discovery in the ubiquitin–proteasome system. Nat Rev Drug Discov 5, 596–613 (2006). https://doi.org/10.1038/nrd2056
Ubiquitin’s role extends far beyond protein degradation. It participates in diverse cellular processes vital for neuronal development, synaptic plasticity, and neuronal signaling. Ubiquitin-mediated proteolysis regulates the turnover of key proteins involved in synaptic transmission, neuronal differentiation, and axonal guidance, shaping the intricate wiring of the nervous system during development.
Moreover, ubiquitin modulation influences synaptic plasticity, the cellular basis of learning and memory. By controlling the stability and turnover of synaptic proteins, ubiquitin dynamically regulates synaptic strength and connectivity, facilitating the formation and remodeling of neural circuits in response to experience.
Schmidt, M.F., Gan, Z.Y., Komander, D. et al. Ubiquitin signalling in neurodegeneration: mechanisms and therapeutic opportunities. Cell Death Differ 28, 570–590 (2021). https://doi.org/10.1038/s41418-020-00706-7
Ubiquitin dysregulation has been implicated in various neurodegenerative disorders, including Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease. In these conditions, aberrant accumulation of ubiquitin-tagged proteins contributes to the formation of pathological aggregates, characteristic of neurodegenerative pathology.
I had the privilege of meeting with Dr. Aaron Ciechanover, a distinguished scientist who was awarded the Nobel Prize in Chemistry for his groundbreaking work characterizing the method by which cells degrade and recycle proteins using ubiquitin. Learning about his work provided me with invaluable insights into the intricacies of ubiquitin-mediated proteolysis and its implications for cellular physiology and disease.

Contents
Newsletter
- Updates from Makliya Notes will be delivered to your inbox.